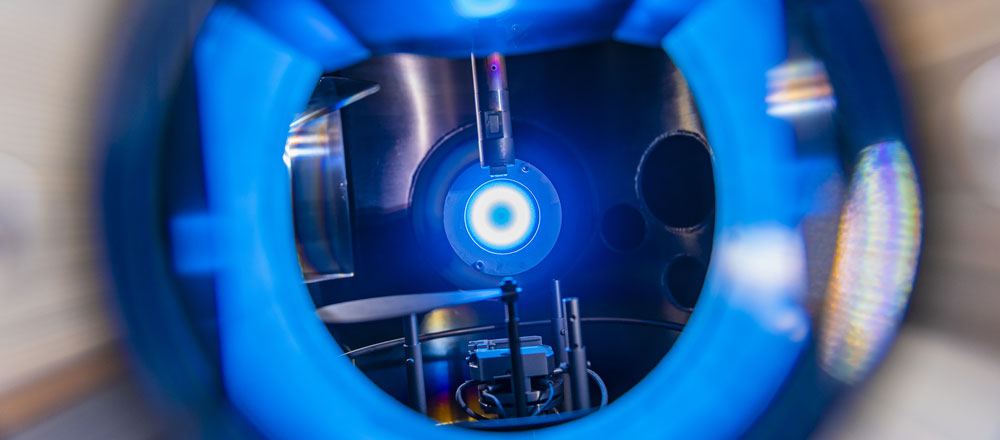
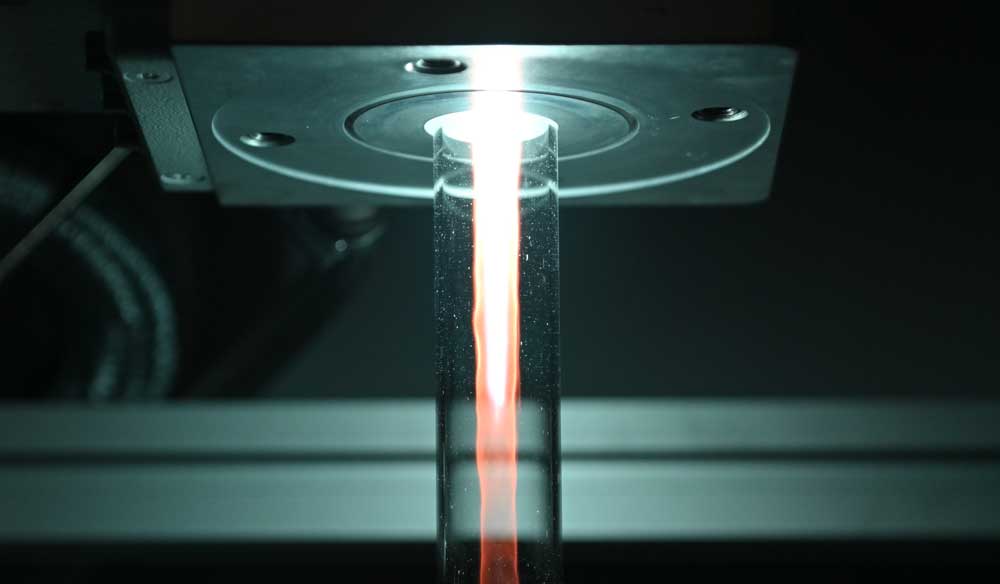
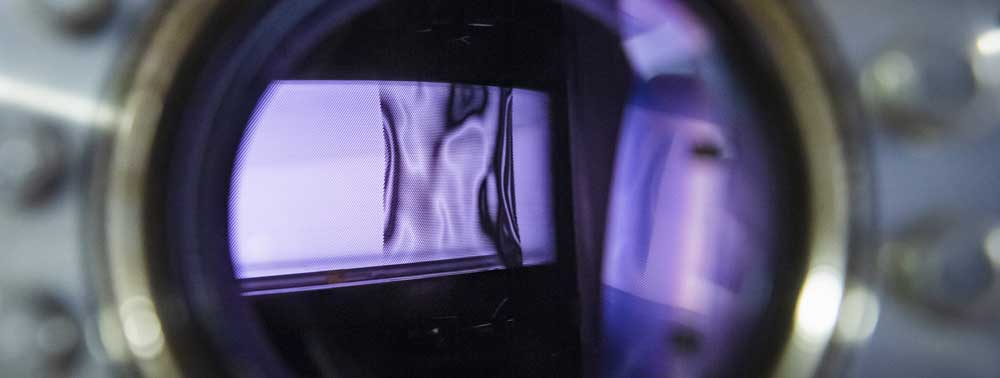
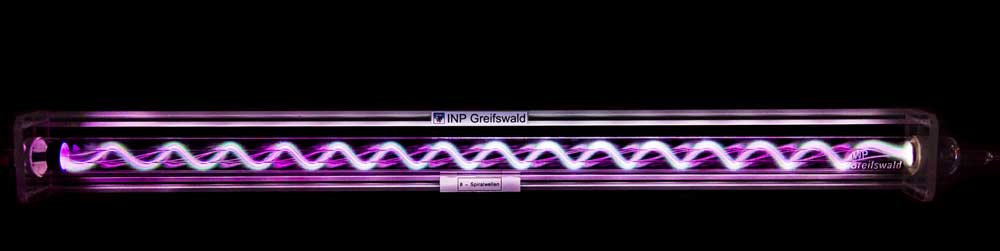
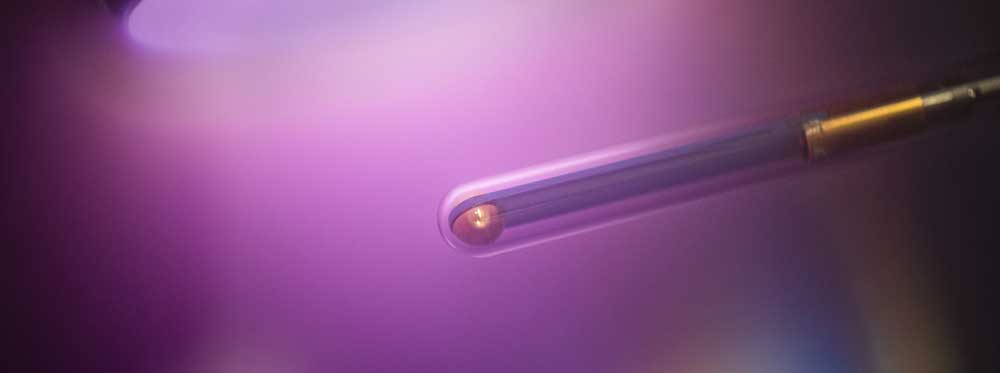
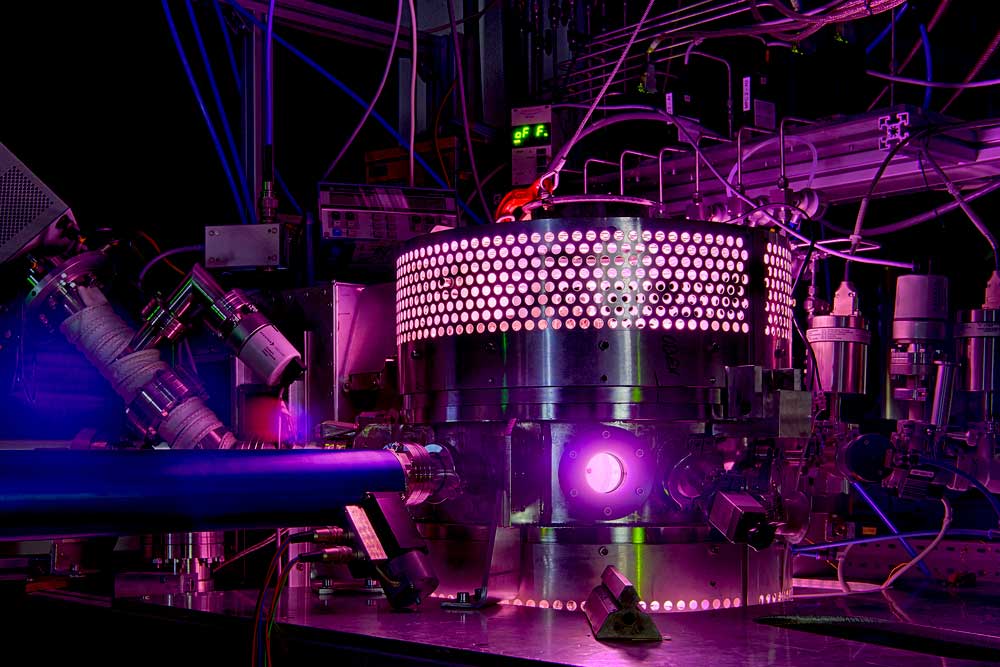
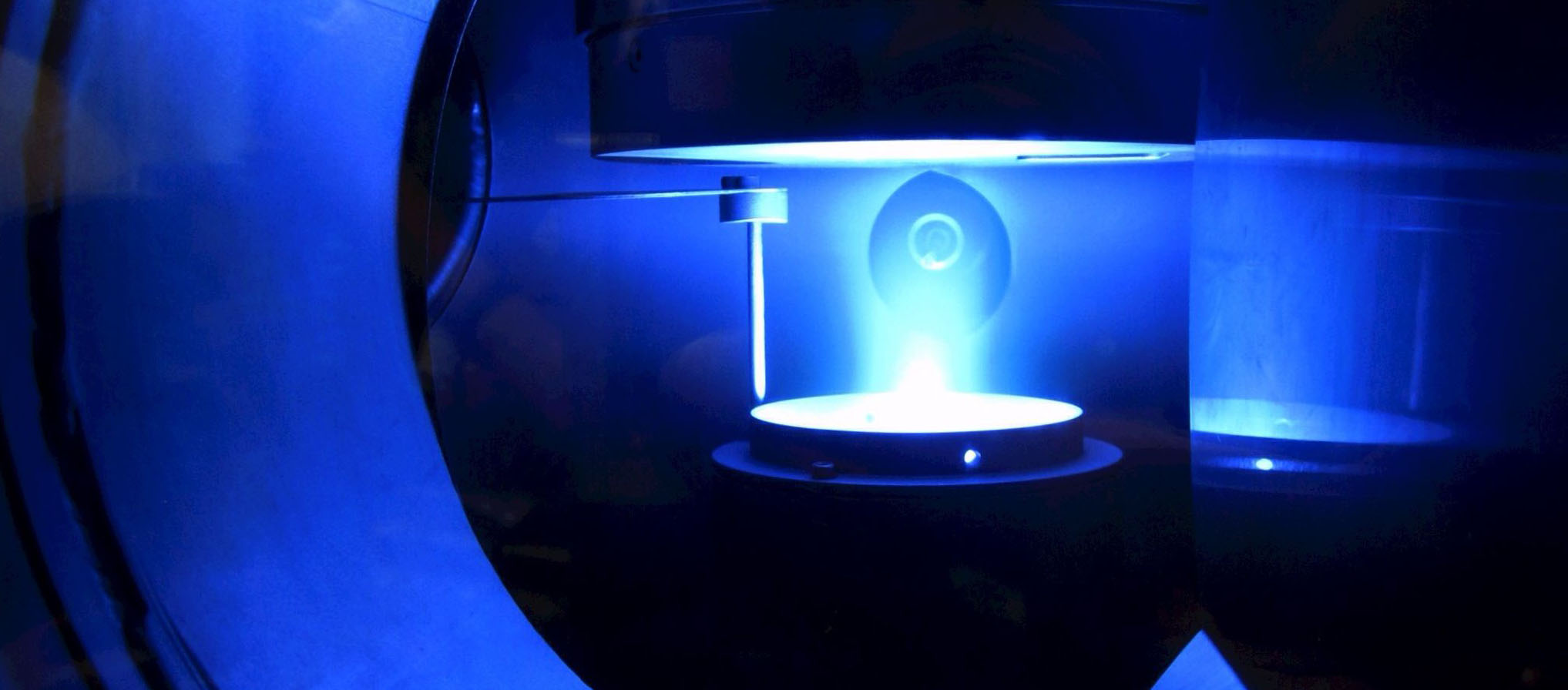
A5 - sDBDSurface Streamer PropagationTime-resolved electron impact excitation rates showing |
- Details
Research Data Management
Exchange Research Data Management UA Ruhr & Oulu
Under the slogan "Mining of research data instead of coal" the UA Ruhr organized an international knowledge exchange on data management with researchers working as data stewards at the University of Oulu, Finland on 11th and 12th May. The event was hosted in the convention center of Ruhr University Bochum and by zoom.
The local structures supporting research data management at the UA Ruhr universities were presented and experiences on advice, training and data management plans, and discuss best practices were shared.
Furthermore, an exchange between the CRCs CRC 1280 and CRC 1316 as well as the partners from UA-Ruhr and the Finnish guests took place. Here researchers and data stewards as well as data experts from the consortia were brought together.
The event was a great success, as the structures on both sides differ, but the challenges of the individual actors in the field of data management are identical. Thus, many interesting experiences could be exchanged. Due to the success, a return visit to Oulu is now being planned to continue the exchange.
- Details
Research Data Management
Data Stewards from Finland visit RUB
An Erasmus program enables exchange among university staff. RUB welcomes guests from the University of Oulu.
RUB receives a visit from Finland. The Erasmus+ Staff Mobility Program enables visits of university staff to expand internationalization. From the UNIC partner university Oulu in Finland, researchers from different faculties are visiting RUB to exchange ideas on research data management.
On May 11, 2022, the so-called Data Stewards will be welcomed at an event hosted by RUB, the University of Duisburg Essen (UDE) and the Technical University (TU) Dortmund at the Event Center on campus. The Data Stewards are responsible for the Faculty of Education, Oulu Business School, Faculty of Humanities, Faculty of Technology and Faculty of Science and Biocenter Oulu.
The participating guests are Data Stewards from various collaborative projects, personnel from the libraries and central IT, and interested researchers from the three universities of the University Alliance Ruhr. In addition to the RUB, these include the UDE and TU Dortmund. On May 12, the program for the Finnish guests will also include one-on-one discussions and roundtable discussions in smaller groups. These include, for example, laboratory tours of the Collaborative Research Centers "Extinction Learning" (SFB 1280) and "Transient Atmospheric Pressure Plasmas - from Plasma to Liquids to Solids" (SFB 1316), a discussion round on the topic of Open Science and talks with the University Library, the WORLDFACTORY Start-up Center and the Materials Research Department.
adapted from Katrin Heyer, RUB
- Details
Awards
Faraday Medal for Beatriz Roldan
FHI Director Beatriz Roldán Cuenya will be awarded the prestigious Faraday Medal from the Royal Society of chemistry of UK for 2022. She will receive the medal at “Electrochem2022” in Edinburgh this September. In 1987, there was another prior winner from FHI, former director and world-wide electrochemistry leader, Prof. Heinz Gerischer.
- Details
Scientific Publications
Achim von Keudell new Editor in Chief for Plasma Processes and Polymers
Achim von Keudell became with the beginning of March one of the four Editors in Chief of Plasma Processes and Polymers.
- Details
MGK Colloquium
Virtual MGK colloqium 2022
With the start of the second funding period, all PhD students of the projects within the CRC 1316 were invited to participate in the annually MGK colloquium. The colloquium was held online at 7th and 8th of March 2022 and was organized by Maximilian Klich (project A8) and David Schulenberg (project A4).
The colloquium served as an introduction of the many new PhDs joining the CRC 1316 in its second funding period. Without the participation of project leaders it achieved a casual atmosphere for sharing scientific knowledge and interests between the PhD students. Here, they could introduce themselves and their projects as well as give a short summary about their projects achievements and future plans through talks or a poster presentation. Leading the talks regarding the different research topics modelling, DBD/RF discharges and plasma in liquids were the invited speakers which generated a well rounded experience.
The new funding period also required a few elections, namely the position of the PhD speaker formerly held by Maximilian Klich, as well as a position in the CRC 1316 gender board held by Lars Schücke (project A7) and Katharina Grosse (project B7). The newly elected PhD speaker is David Schulenberg and Stefanie Bogenrieder (project B4) as well as Lukas Forschner (project B12) are the new gender board representative.
Sascha Chur, project B2 of the CRC 1316
- Details
Research
P. Grosse publishes recent results on electrochemical CO2 reduction in Nature Communications
A team of researchers from the Department of Interfacial Sciences at FHI Berlin has discovered how changes in the structure of copper catalyst particles during electrochemical CO2 reduction affect their catalytic performance. This should lead to the development of new catalysts that convert the greenhouse gas CO2 into useful chemicals. Researchers around CRC member Philipp Grosse from project B1 published their work in the journal Nature communications.
The could show how the initial number of catalysts particles, their size and density on the support electrode surface is not a reliable indicator of the actual number of particles present during reaction. More importantly, Philipp Gosse and colleagues show that by optimizing the design of the pre-catalyst structures, the structural evolution under working conditions is influenced and thus also their selectivity. This is also important for the electron microscopists.
- More details can be found here.
- Details
Working group plasma electrolysis
The Future German and European Energy System – The Role of Hydrogen and its Production, Transport, and Use
A plasma workshop will take place with a special lecture on electrolysis, focusing on future energy scenarios, by Marcel Fiebrandt from Thyssengas on the 25th of February at 10:00.
The EU's Green Deal and the German Federal Climate Protection Act, adapted in 2021, set the ambitious goal of achieving greenhouse gas neutrality by 2050 and 2045, respectively. The necessary transformation of the world's fourth-largest economy toward climate neutrality requires massive adjustments in all sectors and energy carriers as well as in the energy infrastructure. In the national and European hydrogen strategy, hydrogen in combination with renewable energy sources is assigned a central role for the transformation of the European energy system. This is the case as the properties of hydrogen are able to complement the increasing electrification of the sectors. For example, hydrogen can be stored in large underground caverns, transported in pipelines and used for power generation when renewable energy generation is insufficient, and it provides decarbonization opportunities in applications that cannot or are difficult to decarbonize through direct electrification. As a result, power-to-gas-systems, and especially electrolysers, will play a key role as the interface of electricity and gas infrastructure in the future integrated energy system to couple both energy carriers. In this talk, a brief overview of the future energy system scenarios and their implications on the industrial, mobility, and heating sector will be given. Afterwards, the options of producing hydrogen will be discussed and which requirements they have to fulfil to be integrated effectively and economically in the future energy system. Due to their critical role at the interface of the power and gas grid, special attention is paid to the technical requirements based on the properties and restrictions of the energy grid.
- Details
Awards
Heraeus Dissertation Award of the Faculty of Physics and Astronomy at RUB for Dr. Katharina Grosse (project B7)
The generation of plasmas in liquids is important for applications such as electrolysis, water purification or medicine, but also opens up a number of very fundamental questions. These plasmas are generated by short voltage pulses in the range of many kilovolts and a few nanoseconds in duration applied to a tungsten tip submerged in water. There is a lively debate around understanding the ignition of these plasmas, as electron multiplication during plasma ignition is postulated to occur either within small nanovoids, small fractures in the water, or as an electron avalanche in the water itself. In both cases, field emission at interfaces or field ionization of water molecules plays a crucial role. Dr. Grosse studied the whole dynamics of these plasmas from ignition to afterglow using time-resolved emission spectroscopy and compared it with modeling of emission and fluid dynamics. It showed that the broad continuum is produced by blackbody radiation, with a temperature of 7000 K, exactly equal to that of boiling tungsten. Electron densities of several 1025 m-3 can be derived from the strong broadening of the Balmer lines of the hydrogen atoms. Furthermore, a strong self-absorption of light from the region of the plasma channel is observed while light from the running ionization front shows no self-absorption. From this it can be deduced that the plasma runs directly through water and is not formed within nanovoids. Thus, field emission and field ionization dominate. After this first plasma pulse, the high power density leads to the phase transition from water to water vapor and bubble formation within the first microsecond. The high pressure in the range of GPa causes an expansion of the cavitation bubble and the generation of a sound wave propagating in the liquid. This could be directly observed using shadowgraphs. In particular, the speed of sound reaches several 1000 meters per second, indicating the very high pressure at the beginning of the discharge. Based on this measurement, Dr. Grosse has very significantly extended the understanding of these plasmas.
- Details
Award
Two awards for SFB researchers at the Academic Celebration 2021


PhD Student David Steuer recieved an award for his exceptional work during his master degree at the Academic Celebration of the Ruhr-University Bochum on the 24th of November. His thesis „Comparative Investigation of Two-Dimensional Oxygen Distributions in Microplasmas by Optical Methods” has been chosen to be the best master thesis in 2020 from the Faculty of Physics and Astronomy.
Additionally, Dr. Marco Krewing received the GdF-Award for exceptional interdisciplinary work during his dissertation with the title “Impact of low-temperature plasmas on microorganisms and biomolecules”. We congratulate both award winners to this special honor.
Images: M.Sc. David Steuer, Projects A6, B2 (left) and Dr. Marco Krewing, Project B8 (right)