General Questions
Non-equilibrium processes are the basis of a multitude of phenomena in nature such as transport, excitation of atoms and molecules and de-excitation and dissipation at surfaces. The non-equilibrium character of plasmas is especially pronounced due to the high energy density in these systems and the very selective excitation of, for example, only the electrons. If these plasmas are brought into contact with solids or liquids, the non-equilibrium character can be transferred to other states of matter. An excellent example are plasma chemistry processes that are directly coupled to catalytically active surfaces.
The use of non-equilibrium atmospheric pressure plasmas is most interesting since they can most easily be combined with standard chemical processes. The non-equilibrium character of these plasmas can be controlled by large gas flows or by short pulsed excitation assuring strong cooling mechanisms. Thereby, a huge variety of desired plasma chemistries or emission patterns can be adjusted following an empirical strategy. However, any further progress is hampered by the lack of a fundamental understanding of those discharges and their interaction with fluid and solid interfaces leading to many open questions:
- How to reach and maintain a stable atmospheric pressure non-equilibrium discharge in a range of different gas mixtures?
- How to efficiently transport the species from the plasma to the object to be treated or coated?
- What are the chemical non-equilibrium synthesis routes of new materials or species?
- How does the transfer of species and energy occur on the nanosecond timescale?
- What are the roles of gaseous, liquid, biological, and solid state catalysts in contact with those plasmas?
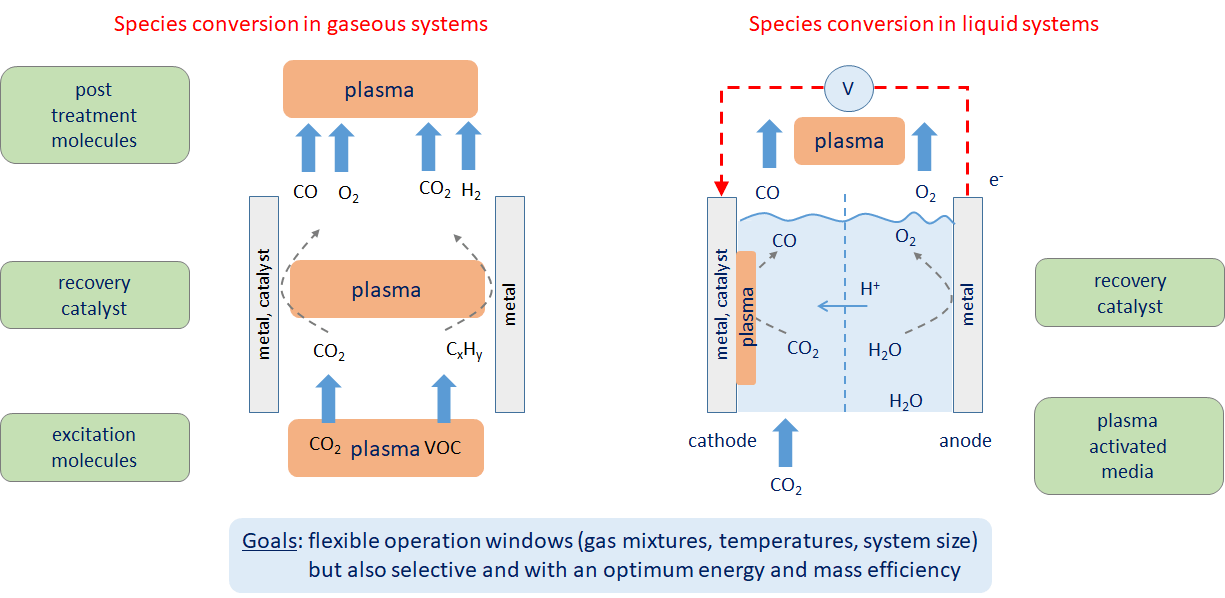
The Collaborative Research Centre (CRC) 1316 “Transient atmospheric plasmas – from plasmas to liquids to solids” addresses these research questions by combining expertise in plasma physics, surface physics, chemistry, biotechnology, and engineering. This CRC focuses on transient atmospheric plasmas at varying spatial and temporal scales for the nanostructuring and activation of catalytic surfaces, for the coupling to catalysis and biocatalysis, as well as for electrochemical processes. Due to the strong interaction between these plasmas and the confining interfaces, special in-situ, real-time, and in-operando methods will be employed. The research program follows three consecutive phases with reaching a basic understanding at the beginning to the optimum integration of plasma and active surface, until the up-scaling of these plasmas. The CRC 1316 seeks optimal solutions for systems for energy conversion (solar fuels, CO2 harvesting, photocatalysis), to health (removal of volatile organic compounds from air streams), for biotechnology (plasma-driven biocatalysis), and for technical chemistry (bottom-up synthesis from small molecules to valuable chemicals).
Projects of the CRC1316
-
A1:
Highly sensitive measurements of electric fields with E-FISH, and key atomic and molecular species with THz absorption spectroscopy and cavity-enhanced spectroscopy techniques
Jean Pierre van Helden, Inna Orel, Uwe Czarnetzki, Nikita Lepikhin
-
A2:
Ro-vibrational distribution measurement in transient discharges by precision frequency comb spectroscopy
Ibrahim Sadiek, Jean Pierre van Helden, Christian Busch, Dirk Luggenhölscher, Uwe Czarnetzki
-
A3:
Excitation transfer between molecules in transient atmospheric pressure plasmas and its impact on plasma chemistry
Achim von Keudell, Steijn Vervloedt, Siqi Yu, Fatma-Nur Seferoglu, Daniel Henze
-
A4:
Process control in micro atmospheric pressure RF plasma jets by voltage waveform tailoring and customised boundary surfaces
Julian Schulze, Lena Bischoff, Yue Liu
-
A5:
From ns- to ms-pulses - dynamics of surface dielectric barrier discharges
Máté Vass, Dominik Filla, Thomas Mussenbrock
-
A6:
Pulsed plasma interaction with catalytic surfaces within micro-structured array devices
Marc Böke, Judith Golda, Henrik van Impel, Yue (Amy) Cheng, David Eiselt (born Steuer), Volker Schulz-von der Gathen
-
A7:
Plasma catalysis for conversion of volatile organic compounds (VOC)
Martin Muhler, Ihor Korolov, Timothy Oppotsch, Gerrit Hübner, Jonas Hiepel, Luisa Lachnicht, Sophie Makhynya , Peter Awakowicz
-
A8:
A 3-dimensional kinetic transport and reaction model of atmospheric pressure plasma jets
Máté Vass, Thomas Mussenbrock, Maximilian Klich, Ralf Peter Brinkmann
-
A9:
Chemical kinetics model for plasma liquid interfaces
Lars Schücke, Thomas Mussenbrock, Youfan He, Ralf Peter Brinkmann, Efe Kemaneci, Andrew Gibson
-
B1:
Liquid plasma electrochemistry: activating catalytic surfaces and driving electrochemical transformations of plasma-activated species
Beatriz Roldan, Philipp Grosse, Sebastian Öner, Khanh-Ly Nguyen
-
B2:
Self-organisation of sub-micrometer surface structures stimulated by microplasma generated reactive species and short-pulsed laser irradiation
Marc Böke, Judith Golda, Sascha Chur, Alexander Schicke, Volker Schulz-von der Gathen
-
B4:
Combined theoretical and experimental studies on the impact of plasma-generated species on surface modifications and catalytic properties of well-defined electrodes and photocatalytic materials
Timo Jacob, Mohammed Elnagar, Björn Kirchhoff, Josef Anton
-
B5:
2D-plasma-liquid-solid interfaces - plasma electrolysis
Ihor Korolov, Lars Schücke, Florens Grimm, Jan-Luca Gembus, Peter Awakowicz, Andrew Gibson
-
B7:
Reaction chemistry of plasmas in liquids interacting with surfaces
Achim von Keudell, Pia-Victoria Pottkämper, Oliver Krettek, Sven Weller
-
B8:
Non-thermal plasma-driven biocatalysis
Julia Bandow, Hanna-Friederike Poggemann
-
B11:
Rational tuning of plasma and liquid chemistry for biocatalysis
Tim Dirks, Judith Golda, Sabrina Klopsch, Anna Lena Schöne, Robin Minke, Steffen Schüttler, Andrew Gibson
-
B12:
Impact of plasma in liquid on electrodestructure and solution properties
Timo Jacob, Lukas Forschner, Albert Engstfeld
-
B13:
Plasma-derived nanocatalysts for hydrogen evolution
Kristina Tschulik, Gabriel Boitel-Aullen, Paolo Cignoni
-
B14:
The solvated Electron at the electrified solid/liquid interface: structure and dynamics from ab initio molecular dynamics simulations
Marialore Sulpizi, Anna Gomes
-
B15:
Plasma-modified ferroelectric catalysts for the plasma-assisted conversion of volatile organic compounds
Bastian Mei, Stephen Fanenstich
-
INF:
Information Infrastructure
Achim von Keudell, Marina Prenzel
-
MGK:
Integrated Research Training Group
Judith Golda, Bastian Mei
-
PR:
Public Outreach
Marina Prenzel, Ida Hülsbusch, Lara Boeddinghaus

